Atomic Mass and Nuclear Binding Energy for Ar-32 (Argon)
Abstract
This document is part of the Supplement containing the complete sets of data of Subvolume A `Nuclei with Z = 1 - 54' of Volume 22 `Nuclear Binding Energies and Atomic Masses' of Landolt-Börnstein - Group I `Elementary Particles, Nuclei and Atoms'. It provides atomic mass, mass excess, nuclear binding energy, nucleon separation energies, Q-values, and nucleon residual interaction parameters for atomic nuclei of the isotope Ar-32 (Argon, atomic number Z = 18, mass number A = 32).
Argon Atomic Mass Rounded

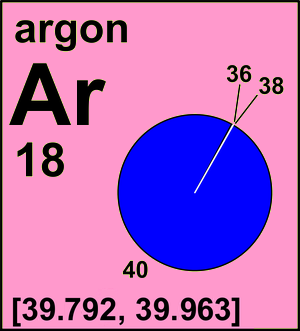
Argon is a chemical element with atomic number 18 which means there are 18 protons and 18 electrons in the atomic structure. The chemical symbol for Argon is Ar. Atomic Mass of Argon Atomic mass of Argon is 39.948 u. Name: Argon: Symbol: Ar: Atomic Number: 18: Atomic Mass: 39.948 atomic mass units: Number of Protons: 18: Number of Neutrons: 22: Number of Electrons: 18: Melting. William Ramsay, in addition to argon, discovered all the noble gases except radon. This earned him the 1904 Noble Prize in Chemistry. The original atomic symbol for argon was A. In 1957, the IUPAC changed the symbol to the current Ar. Argon is the 3 rd most common gas in Earth's atmosphere. Argon is produced commercially by fractional. IUPAC Standard InChIKey: XKRFYHLGVUSROY-UHFFFAOYSA-N CAS Registry Number: 7440-37-1 Chemical structure: This structure is also available as a 2d Mol file; Other names: Ar; UN 1006; UN 1951; argon atom.
- atomic mass;
- mass excess;
- nuclear binding energy;
- nucleon separation energy;
- Q-value;
- nucleon residual interaction parameter
Argon Atomic Structure
The atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, D). The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. (Atomic mass is also referred to as atomic weight, but the term 'mass' is more accurate.)
For instance, it can be determined experimentally that neon consists of three isotopes: neon-20 (with 10 protons and 10 neutrons in its nucleus) with a mass of 19.992 amu and an abundance of 90.48%, neon-21 (with 10 protons and 11 neutrons) with a mass of 20.994 amu and an abundance of 0.27%, and neon-22 (with 10 protons and 12 neutrons) with a mass of 21.991 amu and an abundance of 9.25%. The average atomic mass of neon is thus:
| 0.9048 | × | 19.992 amu | = | 18.09 amu |
| 0.0027 | × | 20.994 amu | = | 0.057 amu |
| 0.0925 | × | 21.991 amu | = | 2.03 amu |
| 20.18 amu |
The atomic mass is useful in chemistry when it is paired with the mole concept: the atomic mass of an element, measured in amu, is the same as the mass in grams of one mole of an element. Thus, since the atomic mass of iron is 55.847 amu, one mole of iron atoms would weigh 55.847 grams. The same concept can be extended to ionic compounds and molecules. One formula unit of sodium chloride (NaCl) would weigh 58.44 amu (22.98977 amu for Na + 35.453 amu for Cl), so a mole of sodium chloride would weigh 58.44 grams. One molecule of water (H2O) would weigh 18.02 amu (2×1.00797 amu for H + 15.9994 amu for O), and a mole of water molecules would weigh 18.02 grams.
Symbol For Argon
The original periodic table of the elements published by Dimitri Mendeleev in 1869 arranged the elements that were known at the time in order of increasing atomic weight, since this was prior to the discovery of the nucleus and the interior structure of the atom. The modern periodic table is arranged in order of increasing atomic number instead.
